The AstraZeneca vaccine: side effects, booster, and Omicron efficiency
In the light of the current COVID-19 situation and related vaccination trends, many countries throughout the globe have begun a gradual relaxation of coronavirus measures. Still, medical authorities affirm the risk remains and encourage further vaccination.
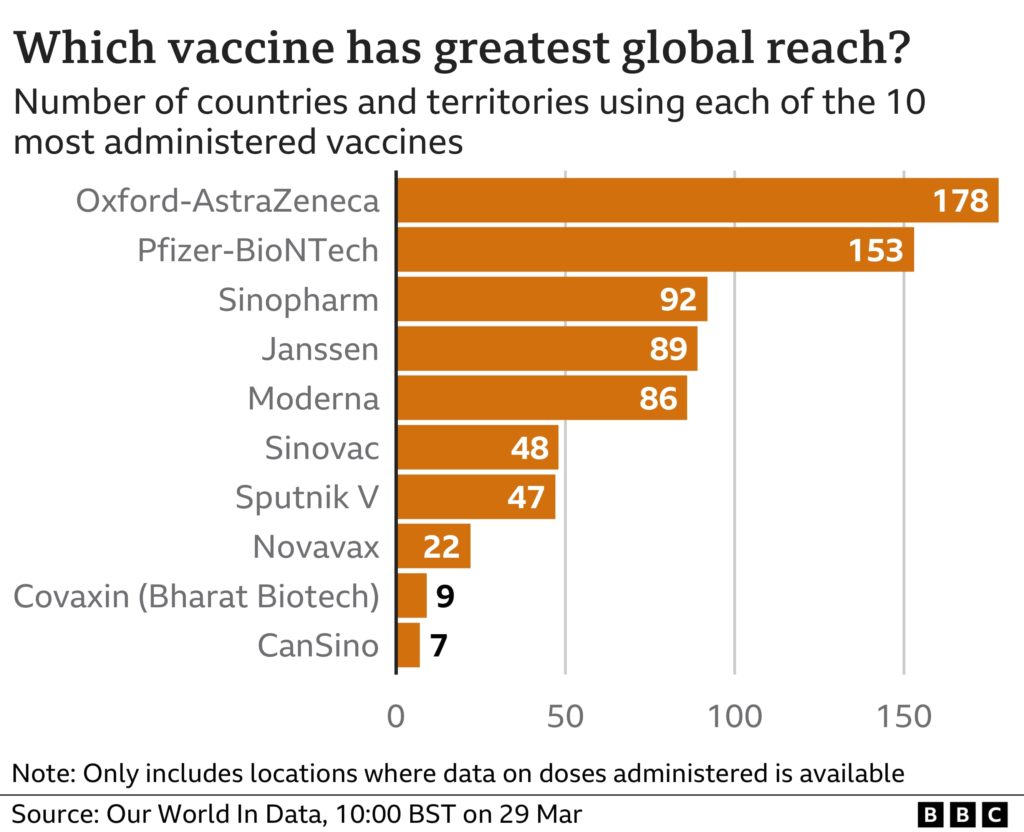
As of April 2022, more than 11.2 billion coronavirus vaccine doses have been administered, in at least 197 countries worldwide. According to Our World In Data, the most used jab worldwide is the Oxford-AstraZeneca viral vector vaccine.
What COVID-19 vaccine does AstraZeneca make?
Ever since the public announcement of the COVID-19 AstraZeneca vaccine, the company name of the UK-based global biopharmaceutical corporation – AstraZeneca (AZN) – serves as a synonym for the vaccine itself. It is, however, the University of Oxford and its spin-out company, Vaccitech, that co-invent the adenovirus vaccine. At a later stage, the two institutions signed an agreement with AZN to lead the manufacture and distribution of the vaccine internationally.
Pascal Soriot, Chief Executive Officer, AstraZeneca, said:
As COVID-19 continues its grip on the world, the need for a vaccine to defeat the virus is urgent. This collaboration brings together University of Oxford’s world-class expertise in vaccinology and AstraZeneca’s global development, manufacturing and distribution capabilities. Our hope is that, by joining forces, we can accelerate the globalisation of a vaccine to combat the virus and protect people from the deadliest pandemic in a generation.
Thanks to the joined forces, AstraZeneca accelerated the globalization of the viral vector vaccine developed by Oxford University’s Jenner Institute work. As a direct result, the AstraZeneca vaccine is known under the Vaxzevria brand name in all of Europe. Under a sub-license agreement, the brand Covishield also refers to the vaccine, only manufactured and supplied by the Serum Institute in India.
How does the vaccine work?
The coronavirus vaccine, codenamed AZD1222, falls under the viral vector vaccines category. Simplified, this means the jab contains a genetically modified common cold virus – adenovirus – which is harmless to people. This non-harmful virus delivers instructions to cells in the human body. Once introduced to the muscles via intramuscular injection, it uses cell machinery to produce a harmful piece of a spike protein.
The spike protein is like the blueprints for the coronavirus found on the surface of the virus causing COVID-19. After the cells produce enough of the protein, they display it on the surface, where the immune system recognizes it as a threat. Next, the immune system produces antibodies and activates other immune cells to fight off what it considers an infection. This response educates our bodies on how to protect us against future contagion with coronavirus.
At no point do viral vector vaccines introduce a harmful virus to the body that could lead to an infection with the COVID-19 virus or with the virus used as a carrier. The genetic material delivered by the vaccine also does not integrate into the DNA. In fact, after vaccination, the spike protein may only last up to a few weeks.
What you need to know about the Oxford-AstraZeneca jab?
This vaccine is available for anyone aged 18 years and older as an intramuscular injection. The initial regimen for AstraZeneca requires two doses with a suggested minimum of 4 weeks in-between each.
The vaccine has been architected by Professor Sarah Gilbert, who holds the Saïd Professorship of Vaccinology. Her chief research interest includes viral vector vaccines that work on the principle of inducing solid and protective adaptive immune responses (T and B cell responses).
According to a press release from Pascal Soriot, CEO of AstraZeneca:
This vaccine’s efficacy and safety confirm that it will be highly effective against COVID-19 and will have an immediate impact on this public health emergency. Furthermore, the vaccine’s simple supply chain and our no-profit pledge and commitment to broad, equitable, and timely access means it will be affordable and globally available, supplying hundreds of millions of doses on approval.
Is the vaccine safe?
As of September 2021, the COVID-19 AstraZeneca vaccine is reported safe and effective at protecting people from coronavirus. The latest vaccine trials account for that with over 34,000 participants.
A specific review of unwanted blood coagulation reactions related to the AZD1222 vaccine debunks the claim with the assistance of an independent neurologist. The independent Data and Safety Monitoring Board looked for factors indicating an increased risk of thrombosis or events characterized by thrombosis among 21,583 participants receiving at least one dose of the vaccine.
Furthermore, data evidence that vaccine toleration is consistent across ethnicity and age. Notably, people over 65 indicated vaccine effectiveness stands at 80%.
Yet, you cannot get the AstraZeneca vaccine if you:
- ever had a severe allergic reaction (anaphylaxis) to the vaccine or its ingredients;
- had a blood clot at the same time as having low levels of platelets (thrombocytopenia) after receiving any COVID-19 vaccine;
- are under 18 years old;
- had Capillary Leak Syndrome (a rare condition causing fluid leakage from small blood vessels).
How did the trials go?
Conforming to the Phase III trial of AZD1222, the coronavirus vaccine has shown to be highly effective in stopping COVID-19 symptoms. Data suggests vaccine effectiveness equals 79% on average at preventing symptomatic COVID-19 and 100% efficacy at preventing severe disease and hospitalization.
Also, the vaccine proves to be cost-effective compared to the Pfizer and Moderna vaccines. Able to remain stable at refrigerator temperatures, AZ is easier to store and distribute, unlike Pfizer jab, which requires subzero temperature storage at -70C.
Side effects of AstraZeneca
Some side effects of the AstraZeneca COVID-19 vaccine may occur that usually do not need medical assistance. These side effects do not last long and will not stop you from having a second dose or going about your daily life.
Not requiring immediate medical attention
In case of any mild unwanted reactions, you can expect such to appear up to 1-2 days after getting your vaccination. As your body adjusts to the medicine, symptoms may go away.
If any of the following effects continue or are bothersome, check with your health care professional.
Common
Vomiting, diarrhea, fever, swelling, redness at the injection site, and low levels of blood platelets occurred in less than 1 in 10 people.
Less common
Enlarged lymph nodes, decreased appetite, dizziness, sleepiness, sweating, abdominal pain, itching, and rash occurred in less than 1 in 100 people. Requiring immediate medical attention
Requiring immediate medical attention
Uncommon adverse effects
Along with its needed effects, the AZ vaccine may cause some unwanted ones. Although not all may occur, if acute adverse reactions or allergies develop, you can expect symptoms to show as early as the first 15 minutes.
Check with your doctor immediately if any of the following appear:
- Shortness of breath
- Chest pain
- Severe or persistent headache
- Blurred vision
- Confusion or seizures
It is advisable to wait for a potentially unwanted initial response at the vaccination center for healthcare workers to take immediate action in case of the above.
Rare adverse effects
Limited cases of dangerous side effects include:
- Capillary Leak Syndrome (CLS);
Symptoms of CLS include rapid swelling of the arms and legs, sudden weight gain, and faint feeling.
- Guillain-Barre syndrome (GBS);
The European Medicines Agency (EMA) linked Guillain-Barre syndrome with the oxford vaccine as a rare immune response. Symptoms of GBS may include pain, numbness, and muscle weakness in the arms and legs, which may progress to the chest and face.
- an increased risk of Thrombosis with Thrombocytopenia Syndrome (TTS).
TTS became a general health concern for people under 30 taking the ChAdOx1-S shot, mainly associated with younger female recipients. In the UK, Joint Committee on Vaccination and Immunisation (JCVI) took precautionary action and changed vaccine advice for the reported age group. The Committee concluded that the risks of the vaccine causing the clotting reaction are slightly overweight than the risk of severe illness from COVID-19 in young people.
During pregnancy
Statistical data for the subject is not enough to support any thesis so far.
Health authorities advise all women breastfeeding, considering pregnancy, or pregnant at any stage to carefully weigh the benefits and the potential risks linked with the vaccine. Pregnant women who have already received the first dose of AstraZeneca can receive either an mRNA COVID-19 vaccine, AstraZeneca, or other, depending on country availabilities.
Check with your doctor if considering the AstraZeneca vaccine and discuss whether any mRNA is the better solution.
Long-term protection effectiveness of AstraZeneca
In the early days of the first COVID vaccines, Prof Tim Spector launched the ZOE COVID Study app to collect data and study the symptoms of COVID-19. Professor Tim Spector, a leading scientist at King’s College London and founder of ZOE, gathered data from over 4 million in what turned out to be the largest COVID science project.
In late May 2021, after crunching more than a million entries of data of double vaccinated contributors and over 73,000 unvaccinated ones, the ZOE team compiled a report to reveal how long vaccinations stand effective.
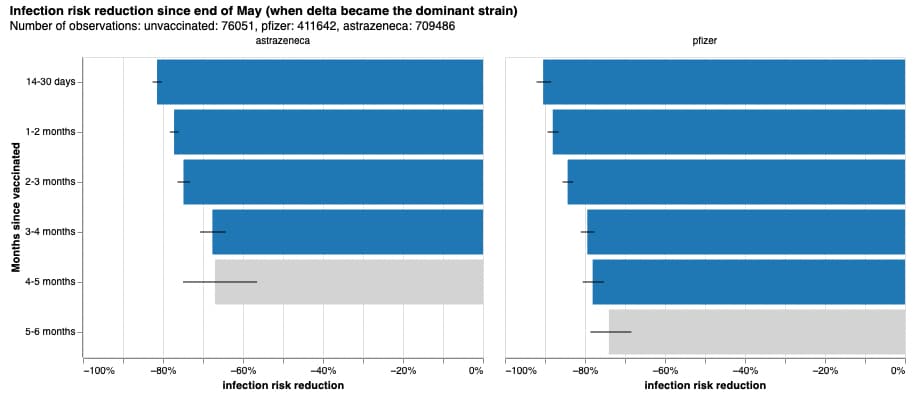
Results testify to the idea that effectiveness wanes over time. In the Oxford-AstraZeneca case, vaccine protection fades to 68% after 3-4 months following a second jab. Findings remind the need for continuous action as new coronavirus variants keep rolling out and cases remain high.
AstraZeneca vs. Omicron
Interim data suggests that primary immunization with AstraZeneca is insufficient at preventing the Omicron variant. Especially from the 15th week onwards, when overall efficacy wanes with time.
Several ongoing trials since the beginning of 2022 support that booster immunization affords better protection against newer SARS-CoV-2 variants. However, vaccine effectiveness against Omicron increased from booster shots, but only to a range of 40%-60%. Comparison with statistics against the Delta variant shows that a third dose increases protection to 95% on average with all combinations. Still, real-world data on vaccine effectiveness against newer SARS-CoV-2 variants remain limited, but booster shots allow for a quick modification to keep up with new coronavirus subsets.
AstraZeneca CEO, Soriot, parred the booster recommendations while urging caution on COVID booster doses. Following his advice, a third dose might not be necessary for everyone.
Dr. Anna Goodman, an infectious disease consultant working on booster vaccine trials, indirectly supports the cautious approach toward booster immunization. In a webinar for the ZOE COVID Study project, she said:
You might want to take different amounts of care depending on your situation and the infection rates in the population.
Booster shot compatibility after AstraZeneca
If you consider getting a booster jab following an initial regimen of AstraZeneca, it is possible to go with any mRNA vaccine of choice.
AstraZeneca is already allowed for usage as a booster, but it is not preferred and suggested. Still, you can use it as a booster in the following situations where you:
- are contraindicated to or had an unwanted adverse event from mRNA vaccines, like a history of anaphylaxis or myocarditis attributed to an mRNA vaccine;
- refuse a preferred vaccine.
AstraZeneca challenges with the vaccine
The COVID-19 pandemic began in late 2019 and quickly spread throughout the world as we know it. That unexpected outbreak brought strict government supervision, including border closures, lockdowns, mask mandates, and social distancing policies.
There was a rush to figure out the best way to overcome COVID-19, not only for the lives at risk but for the economic and social impact that was becoming drier as time progressed.
Experts deduced that the best way to overcome the COVID-19 pandemic was to achieve collective immunity through vaccination with the least amount of damage.
Time and social pressure
It is essential to understand the circumstances under which the vaccine was developed to approach the challenges that the AstraZeneca COVID-19 vaccine faces.
While regular vaccines take approximately ten to fifteen years to develop, the early COVID-19 AstraZeneca vaccine took less than a year. Precautions and safeguarding steps, usually associated with vaccine trials, were shortened. Fears that the vaccine may not be as effective or safe as possible were present, but risks were taken to reduce the death rate.
As a result, the Oxford-AstraZeneca venture received approval for the vaccine in January 2021. Despite being among the first to release a COVID-19 vaccine, co-inventors of the AZ vaccine were also among the first to see notable unwanted effects. Merely two months after the approval for public usage in the UK, incidences of blood clots linked to the vaccine brought the company to public scrutiny.
Blood clots scandal
In March 2021, links to patients developing blood clots following a COVID-19 infection appeared. Soon after, the AstraZeneca COVID-19 vaccine got associated with the phenomenon as a rare side effect.
The European Medicines Agency (EMA) conclusion includes the statement that in rare cases, antibodies that the body produces as a side effect of the vaccine lead to uncontrolled activation of platelets. The direct result of that phenomenon leads to rare cases of the vaccine leaking into the bloodstream which causes a collection of blood cells and coagulation proteins that clump together. Forming a gel-like substance in the blood system, those blood clots start obstructing the blood flow. Sometimes, this might lead to strokes and heart attacks.
This in turn started a scientific hunt to figure out what was going on and if it could be prevented. The UK government commenced an emergency research project with the joined forces of AstraZeneca’s scientists and the Cardiff team.
From the data released, the research report explained two main things:
- Blood clots linked with the COVID-19 infection more so, and to a lesser distinct with some coronavirus vaccines;
- The research investigated two initial clues for the rare blood clots:
- People with clots had unusual antibodies that were attacking a protein in their blood called platelet factor four.
- The greater risk of clots was seen only with some of the vaccine technologies;
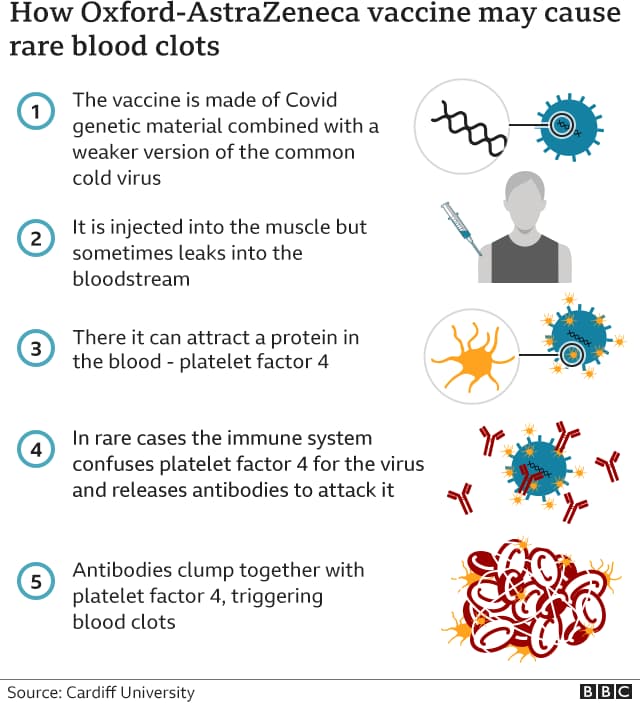
The initial investigation also uncovered that vaccine-induced thrombotic thrombocytopenia (VITT) is not associated with the Moderna or Pfizer-BioNTech mRNA vaccines.
AstraZeneca news coverage
We should note that there was substantial media coverage of these rare events, that shaped the global perception of the vaccine over a night.
Some countries, such as Denmark, have since stopped the usage of the AstraZeneca adenovirus vaccine. Others conducted their trials and found no such risk. The UK changed advice for young people claiming the dangers of the unwanted vaccine response outweigh the possibility of severe coronavirus infection. Ultimately, there has been no consensus on the extent of the side effects of AstraZeneca.
Later on, major global agencies – WHO, EMA, CDC – reported that confirmed cases of VITT in connection to the AZD1222 vaccine are rare. Yet, the UK statistics accounted for 73 deaths out of 50 million doses.
You could never have predicted it would have happened and the chances are vanishingly small, so we need to remember the bigger picture of the number of lives this vaccine has saved
Prof Parker at Cardiff University.
AZN outcome
What is known for sure is that AstraZeneca’s vaccine has proven helpful in limiting the spread and severity of COVID-19 and that many still opt for it when given a choice.
Furthermore, data since the first reports have helped doctors learn how to recognize and treat VITT. Health authorities now know that the condition requires a series of unlucky events, and it is treatable.
